New technologies in compliance with compliance: Blockchain, Saas, Cloud, DevOps, Industry4.0, UX …

Tuesday May 8 2018
Blend Tower, Piazza IV Novembre 7, Milan (Central Station area)
Chairman: Enzo Maria Tieghi – ISPE, CEO ServiTecno
Co-Chairman: Andrea Provini – Aused, CIO Bracco Imaging
DOWNLOAD THE REGISTRATION FORM
Abstract:
In recent years, CIOs and QA/QC and automation/OT systems managers of manufacturing companies, in many industrial sectors, have begun to experiment and adopt new technologies and architectures.
In the pharmaceutical industry the brake of compliance requirements and regulations sector, combined with the recent focus on “Data Integrity”, they braked the adoption of innovations such as Cloud, Mobile, SaaS, Blockchain, UX, Big Data, Analytics, IoT, IIoT, Augmented Reality/Virtual Reality and others.

However, in the Life Science sector today we see a new ferment towards IT / OT technologies and that is why ISPE Italy Affiliate with the contribution of AUSED organizes this Study Day with the collaboration of GAMP Forum Italia, and with the patronage of AFTI, ANIPLA, ASSINTEL, CSA Italy, PDA Italy, in which there will be an opportunity to present and discuss the state of the art of ICT and OT technologies available today, already used or which could be used shortly for the digitization of pharmaceutical companies, also in the critical GxP area.
Although the thrust of the Industry 4.0 plans provides a further stimulus thanks also to tax benefits, it runs the risk of being held back by needs dictated by compliance by the managers of Quality Assurance, Validation, Qualification and Safety of Pharmaceutical Companies.
Just about this ISPE and GAMP have activated various working groups to produce documents and guidelines to ensure that innovation does not conflict with compliance with regulations and to support those who have to develop and validate IT/OT systems.
Among the speakers of the day we will have experts and professionals from industry and academia, validation experts and GAMP, from Regulatory sector, analyst, plant and machinery manufacturers and especially end users/ manufacturers of drugs, biotechnologies and medical devices who will bring their experiences and points of view to be able to compare in the final debate open to all participants.
DOWNLOAD THE REGISTRATION FORM
The program
below is the complete program of the day with all the topics and interventions defined.
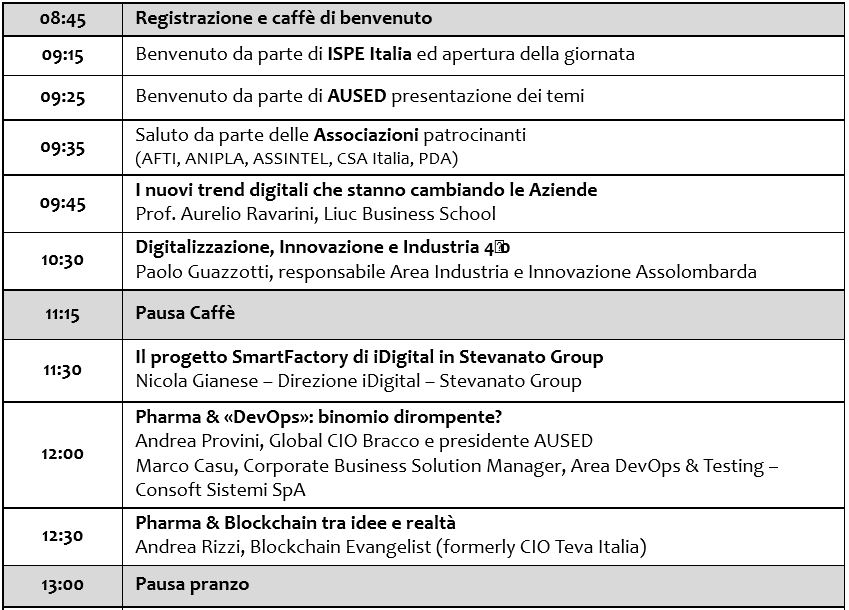
Morning programme
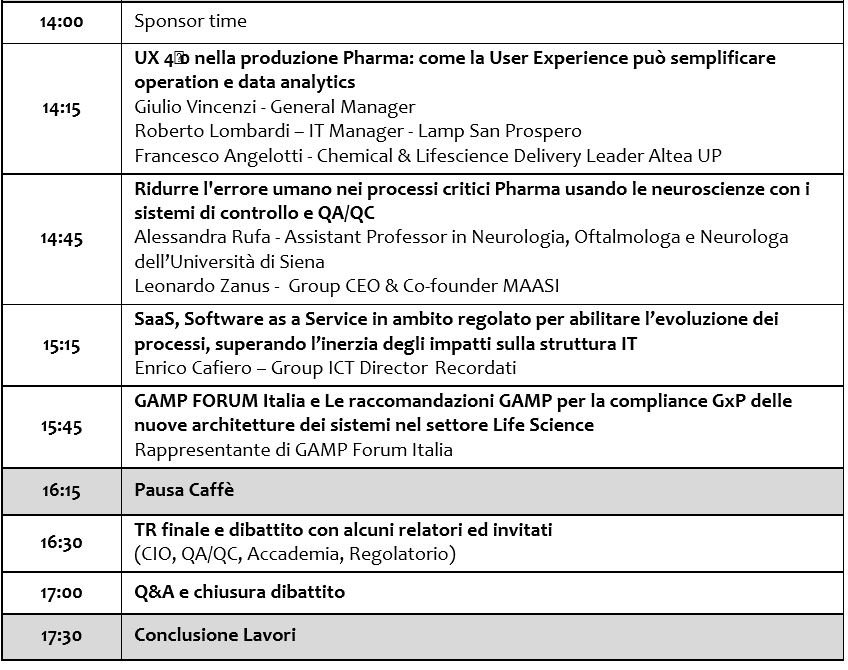
Afternoon programme
How to register

Notes: The ISPE membership fee valid for one year from the date of the event is included in the “Non ISPE Members” registration fee. *Young Professionals are those who have no more than 4 years of work experience. All ISPE members are entitled to discounted prices for the ISPE guidelines, for ISPE events, including international ones, and a free subscription to the Pharmaceutical Engineering magazine. The Secretariat will send a pro-forma letter with the instructions for the payment to be made by bank transfer. Any cancellations must be communicated at least 5 days before the event. Failure to comply with this deadline will result in the entire registration fee being charged. However, substitution with another person indicated by the original member is permitted. ISPE is a non-profit association and therefore is not required to manage VAT; the costs indicated are net, and no invoice is issued but, against payment, a receipt will be sent.
DOWNLOAD THE REGISTRATION FORM